Abstract
Introduction: Unscheduled ER/hospital visits with NSCLC patients (pts) receiving Doc frequently result from complications due to DIN (Patel et al. DOI: 10.1200/JOP.2014.001502). Reducing this healthcare system "touches" could minimize pt inconvenience, financial toxicity, healthcare utilization, and iatrogenic COVID-19. Plin is a novel small molecule that protects bone marrow progenitor stem cells and is non-inferior to pegfilgrastim for the prevention of DIN (Blayney, JAMA Open 2021). In contrast to pegfilgrastim, Plin is given on the same day of chemotherapy (as a single dose per cycle), has minimal bone pain and thrombopenia, has anti-cancer efficacy, and has the potential to minimize healthcare system touches (Blayney, JAMA Onc 2020). Doc 75 mg/m2 in NSCLC pts is typically used without G-CSF prophylaxis ('no treatment'). Here we report on the effectiveness of Plin vs. control for the prevention of DIN in NSCLC from two randomized cancer trials Phase (Ph) 2 Study 101 (NCT00630110) and Ph3 Study 103 (NCT02504489) with positive Overall Survival (Han et al. ESMO 2022). In these studies, absolute neutrophil counts (ANC) were evaluated as a predefined safety- or secondary- endpoint.
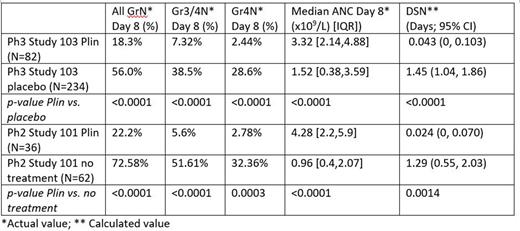
Methods: NSCLC pts received Doc 75 mg/m2, with Plin (20 mg/m2 or its pharmacokinetic equivalent exposure) or no treatment/placebo as control. Pts with primary G-CSF prophylaxis were excluded. ANC blood sampling was predose and day 8 (which approximately coincides with the day of ANC nadir for Doc without or with Plin) to obtain all grade (Gr) neutropenia (N), Gr3/4N, Gr4N frequency, and ANC 'nadir'. Cycle 1 Day 8 ANC ('nadir') was used to calculate DSN since ANC nadir and DSN are strongly correlated. For Plin+ Doc, DSN was derived by imputing ANC nadir into an 'ANC Nadir vs. DSN equation': (DSN=exp (2.82-7.23 x ANC Nadir); r=0.953, p<0.0001) that was calculated from actual ANC nadir and actual DSN using serial ANC values on days 0, 5, 6, 7, 8,9, 10, 15, 21 in NSCLC pts (Blayney JAMA Open Network 2021) from a separate DIN study with Plin (20 mg/m2 or its equivalent of 40 mg fixed dose) and Doc 75 mg/m2 in NSCLC pts (Study 105 NCT03102606). For control (no treatment/placebo) + Doc, DSN was derived by imputing ANC nadir into an 'ANC Nadir vs. DSN equation': (DSN=exp (2.92-8.0 x ANC Nadir); r=0.953; p<0.0001) that was calculated from ANC nadir and DSN obtained from published serial ANC-over-time from data cancer pts (including NSCLC) receiving Doc (Quartino; Invest New Drugs 2015).
Results: Baseline demographics were comparable between the Plin and control in randomized studies 101 and 103. Neutropenia was most prevalent in cycle 1. Cycle 1 ANC results are summarized for studies 101 and 103 for Plin and control (no-treatment/placebo) in NSCLC pts with Doc.
Conclusion: In two independent randomized studies 101 and 103, Plin demonstrated a superior benefit for Gr4N, Gr3/4N, AllGrN, and DSN compared to control (no-treatment/placebo) for Doc-induced neutropenia. The same-day dosing and avoidance of neutropenia with Plin are expected to minimize healthcare touches in NSCLC pts receiving Doc, representing a distinct advantage considering the COVID-19 pandemic.
Disclosures
Blayney:BeyondSpring Pharmaceuticals, In: Other: Principle Investigator. Ogenstad:BeyondSpring Pharmaceuticals, Inc.: Consultancy. Legaspi:BeyondSpring Pharmaceuticals, In: Current Employment. Duprez:BeyondSpring Pharmaceuticals, Inc.: Current Employment. Huang:BeyondSpring Pharmaceuticals, In: Current Employment. Mohanlal:BeyondSpring Pharmaceuticals, Inc.: Current Employment.
Author notes
Asterisk with author names denotes non-ASH members.